Intermolecular Forces: Ion-Ion, Dipole-Dipole, and London Forces.
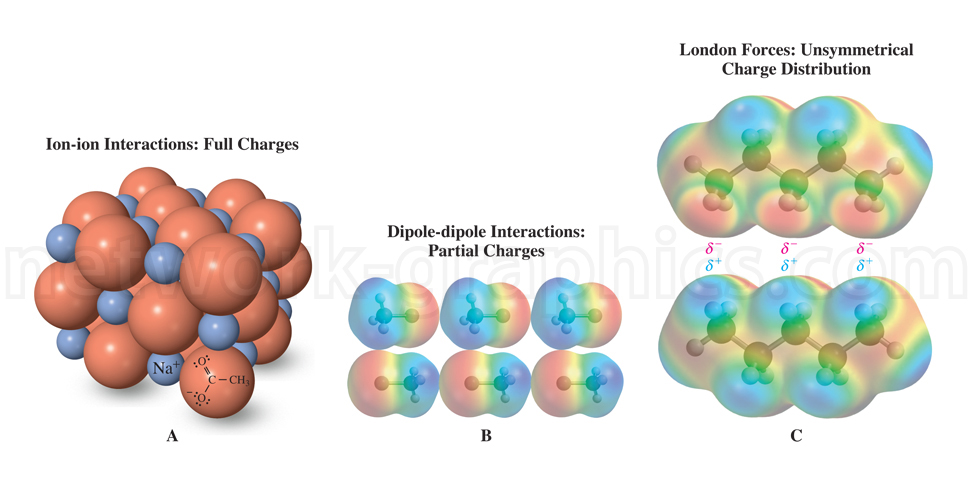
The image compares three types of intermolecular forces. In view A (left), ion-ion interactions are shown between full charges, with sodium ions (Na⁺) interacting with a carboxylate group (–O⁻). This interaction is represented by orange spheres symbolizing the charged ions. In view B (center), dipole-dipole interactions are depicted with partial charges in polar molecules. The colored gradients highlight the regions of partial positive (δ⁺) and partial negative (δ⁻) charges interacting across molecules. In view C (right), London dispersion forces are shown as weak interactions arising from temporary, unsymmetrical charge distributions in nonpolar molecules. The charge distribution is visualized with colored gradients, emphasizing the temporary dipole moments.
This illustration is ideal for chemistry textbooks that cover molecular behavior, states of matter, and bonding theories in physical chemistry.
We can provide sample images or create custom illustrations tailored to your projects. If you are looking for an illustration of this type, or from another subject area, you can contact us to discuss your needs.
Network Graphics / Division of Abramson & Wickham Graphics Inc.
All rights reserved.

