Eclipsing and Angle Strain in Cyclopropane Molecule.
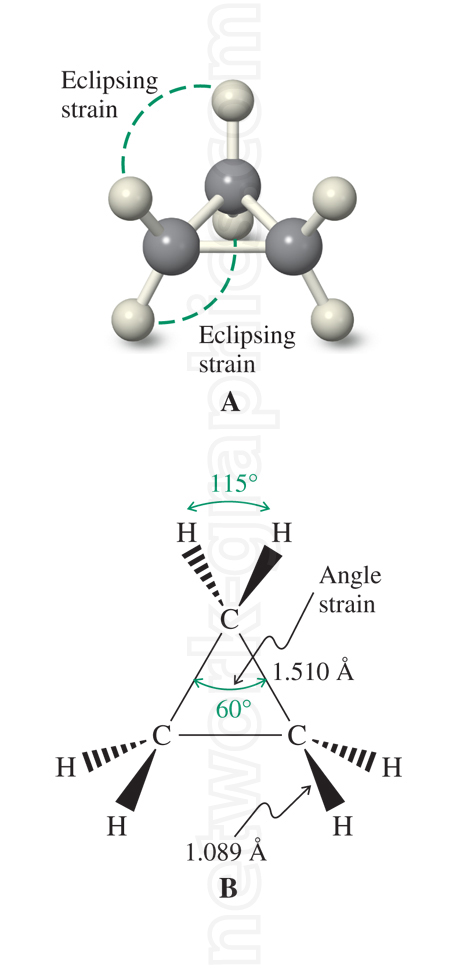
The image shows the cyclopropane molecule with two distinct views. In view A (top), the eclipsing strain is emphasized, illustrating the spatial overlap of hydrogen atoms on adjacent carbon atoms.
This strain results from the close proximity of these atoms, creating torsional strain due to the alignment of bonds. In view B (bottom), the angle strain in the cyclopropane ring is depicted, showing the smaller-than-ideal 60° bond angles between carbon atoms, compared to the optimal 109.5° for sp3 hybridized carbon atoms.
The bond lengths are also labeled, highlighting a C–C bond length of 1.510 Å and a C–H bond length of 1.089 Å.
This illustration is useful for organic chemistry textbooks that focus on structural isomerism, reaction mechanisms, and conformational analysis in hydrocarbons.
We can provide sample images or create custom illustrations tailored to your projects. If you are looking for an illustration of this type, or from another subject area, you can contact us to discuss your needs.
Network Graphics / Division of Abramson & Wickham Graphics Inc.
All rights reserved.

