Electroplating Process Diagram for Chemistry Education.
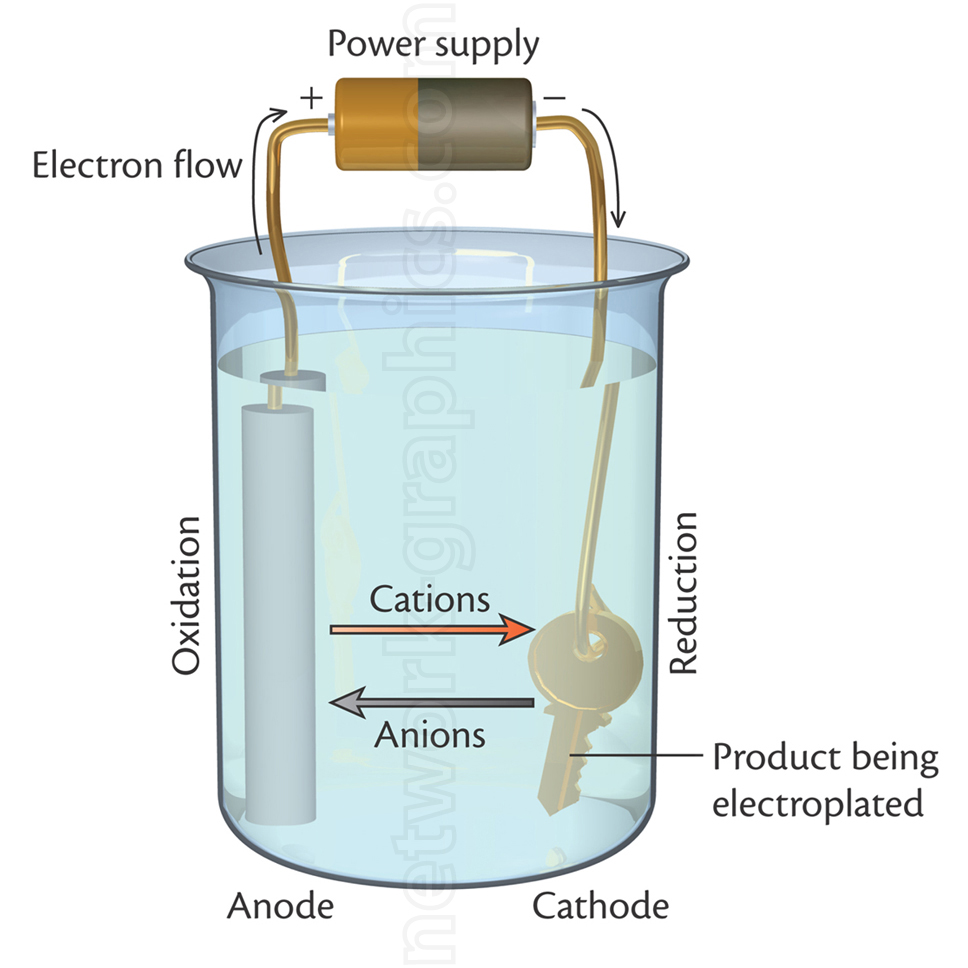
This illustration demonstrates the electroplating process, showing the setup of a key being coated with a metal layer through an electrochemical reaction. The diagram includes a power supply connected to an anode (oxidation site) and a cathode (reduction site), which is the key in this example. Electrons flow from the anode to the cathode, causing cations to migrate towards the cathode for reduction and plating. Anions flow in the opposite direction to maintain charge balance.
This image is highly relevant for chemistry textbooks, particularly those covering topics like electrochemistry, redox reactions, and industrial applications of electroplating.
We can provide sample images or create custom illustrations tailored to your projects. If you are looking for an illustration of this type, or from another subject area, you can contact us to discuss your needs.
Network Graphics / Division of Abramson & Wickham Graphics Inc.
All rights reserved.

